2017-1-11
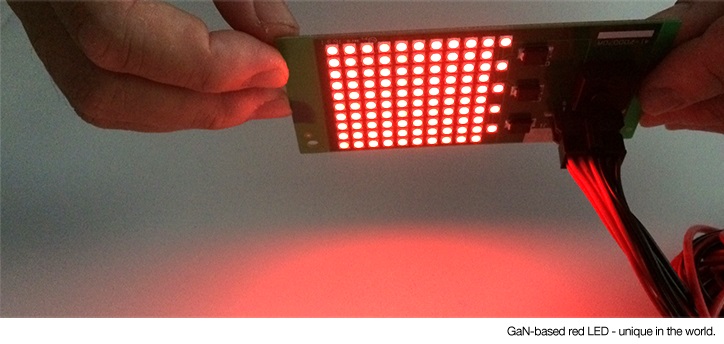
Nitrides are materials that contain nitrogen with a formal oxidation state of minus three. This endows them with high lattice energy, resulting in the appearance of desirable properties such as high hardness for use as coatings, wide bandgaps for application in devices, and hydrogen absorption capability to allow hydrogen storage. Nitride devices have garnered considerable attention for next-generation optical applications such as solid-state lighting.
The performance and lifetime of nitride devices is generally negatively affected by the presence of oxygen, which is difficult to exclude during device fabrication. Nitride devices are often intentionally doped with other ions to modulate their performance. A typical combination is gallium nitride doped with rare earth europium ions, which display stable red emission. However, the effect of oxygen on europium ions doped in gallium nitride was unclear. Recently, an international collaboration between Osaka University researchers and colleagues from the United States and Portugal clarified the role of oxygen in europium-doped gallium nitride devices.
The researchers doped gallium nitride with europium precursors with and without oxygen in their structures. Co-doping gallium nitride with the two types of europium precursors allowed the oxygen content of devices to be carefully regulated. They found that removing oxygen from the devices caused the location of the europium ions in the gallium nitride lattice to shift. That is, oxygen was necessary for the europium ions to stably locate at gallium sites in the crystal lattice. In addition, removing oxygen resulted in precipitation of europium at the device surface.
These structural changes affected the performance of the devices. When the oxygen content was too low, detrimental effects like broad emission peaks and low energy transfer efficiency were observed. Increasing the oxygen content in the devices to a certain degree caused the emission peaks to sharpen and energy transfer efficiency to increase; that is, device performance improved. However, oxygen degrades the performance and reliability of gallium nitride.
“We needed to find the trade-off between minimizing oxygen content to maintain the performance of gallium nitride and having sufficient oxygen for optimal emission from europium,” says Yasufumi Fujiwara from Osaka University's Graduate School of Engineering, Division of Materials and Manufacturing Science.
To reach this trade-off, the researchers fabricated devices containing layers of undoped gallium nitride and europium-doped gallium nitride without intentional oxygen doping of different thickness. They found that decreasing the amount of europium ions (and thus oxygen content) in this manner did not degrade emission performance. This is because the native oxygen in the gallium nitride layers was sufficient to allow the europium ions to locate at gallium sites and thus display favorable emission when the europium-doped gallium nitride layer was sufficiently thin.
“These layered structures exhibited sharp europium-based emission and contained device-compatible oxygen levels,” explains Brandon Mitchell from the University of Mount Union (Ohio, USA). “This approach is promising to optimize the performance of nitride devices doped with rare earth ions.”
The ability to achieve desired dopant emission with a suitably low oxygen content will contribute to the development of advanced solid-state devices for lighting applications.
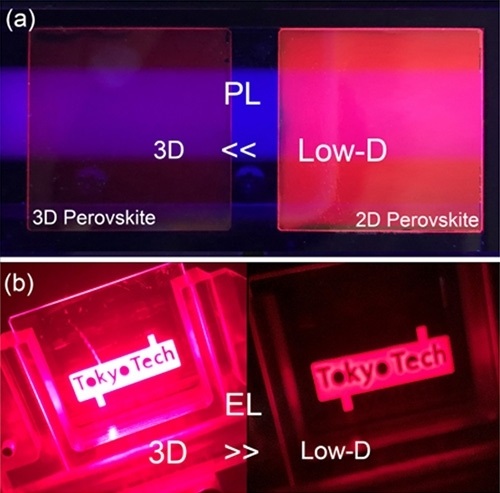
Fujiwara is also in a collaboration with Tom Gregorkeiwicz from the University of Amsterdam (The Netherlans) investigating the bandgap energies of perovskite nanocrystals, another type of material attractive for use in optoelectronic devices. Perovskite nanocrystals display bright luminescence, the color of which can be tuned by structure modulation of the nanocrystals. The collaboration used an advanced scanning transmission electron microscopy technique to examine how the bandgap energy of the perovskite cesium lead bromide depended on nanocrystal size and shape. They determined the relationship between the bandgap energy and size of cesium lead bromide nanocrystals, finding that smaller nanocrystals had a wider bandgap because of quantum confinement. The teamʼs findings will aid the development of custom-designed quantum structures.
Reference:
- 1. B. Mitchell, D. Timmerman, J. Poplawsky, W. Zhu, D. Lee, R. Wakamatsu, J. Takatsu, M. Matsuda, W. Guo, K. Lorenz, E. Alves, A. Koizumi, V. Dierolf & Y. Fujiwara, “Utilization of native oxygen in Eu(RE) – doped GaN for enabling device compatibility in optoelectronic applications,” Scientific Reports 6, 18808 (2016). DOI: 10.1038/srep18808
- 2. Junhao Lin, Leyre Gomez, Chris de Weerd, Yasufumi Fujiwara, Tom Gregorkiewicz, and Kazutomo Suenaga, “Direct Observation of Band Structure Modifications in Nanocrystals of CsPbBr3 Perovskite,” Nano Lett. 16, 7198 (2016). DOI: 10.1021/acs.nanolett.6b03552
This research project was supported by the Osaka University International Joint Research Promotion Program, which aims to further enhance research quality and promote globalization at Osaka University through advanced research with overseas collaborators. Professor Fujiwara jointly conducted this research with the following researchers: Assistant Professor Brandon Mitchell, University of Mount Union, and Professor Tom Gregorkiewicz, University of Amsterdam.