February 21, 2017
When John Crocker, a professor of chemical and biomolecular engineering in the University of Pennsylvania’s School of Engineering and Applied Science was a graduate student, his advisor gathered together everyone in his lab to “throw down the gauntlet” on a new challenge in the field.
Someone had predicted that if one could grow colloidal crystals that had the same structure as carbon atoms in a diamond structure, it would have special optical properties that could revolutionize photonics. In this material, called a photonic bandgap material, or PBM, light would act in a way mathematically analogous to how electrons move in a semi-conductor.
“The technological implication is that such materials would allow for the construction of 'transistors' for light, the ability to trap light at specific locations and build microcircuits for light and more efficient LEDs and lasers,” Crocker said.
At the time, Crocker decided to pursue his own projects, leaving the pursuit of PBMs to others.
Twenty years later, Crocker’s own graduate student Yifan Wang produced this elusive diamond structure while working on a different problem, serendipitously. This put them on the path to achieving PBMs, the “holy grail of directed particle self-assembly,” Crocker said.
“It's a classic story of serendipity in scientific discovery. You can't anticipate these things. You just get lucky sometimes and something amazing comes out.”
The research was led by Crocker, Wang, professor Talid Sinno of SEAS and graduate student Ian Jenkins. The results have been published in Nature Communications.
To be a PBM, a material needs to have a crystal-like structure not on the scale of atoms but on the lengthscale of the light wavelength.
“In other words,” Crocker said, “you need to sculpt or arrange some transparent material into an array of spheres with a particular symmetry, and the spheres or holes need to be hundreds of nanometers in size.”
Back in the 1990s, Crocker said, scientists believed there would be a lot of different possible ways to arrange the spheres and grow the needed structure using colloid crystals similar to how crystals of semi-conductors are grown: colloidal spheres spontaneously arranging themselves into different crystal lattices.
Opals are a natural example of this. They are formed when silica in groundwater forms microscopic spheres, which crystallize underground and then become fossilized in solids.
Although opals don’t have the right symmetry to be PBMs, their iridescent appearance results from their periodic crystal structure being on scales comparable to the wavelength of light.
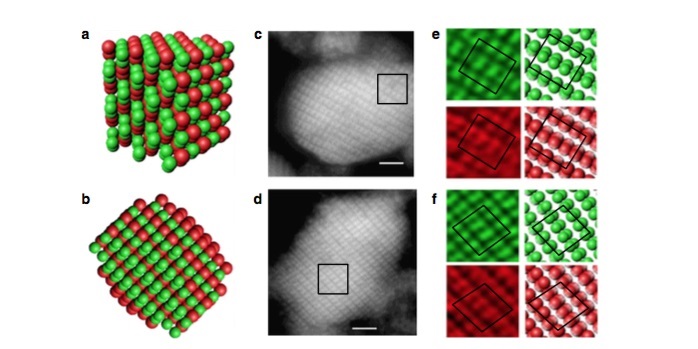
To form a PBM, the major goal is to arrange transparent microscopic spheres into a 3-D pattern that mimics the atomic arrangement of carbon atoms in a diamond lattice. This structure, unlike other crystals, lacks certain symmetry directions of other crystals where light can behave normally, allowing the diamond structure to maintain the PBM effect.
Scientists assumed they would be able to make synthetic opals with different structures using different materials to produce PBMs. But this proved more difficult than they had thought and, 20 years later, it still hasn’t been accomplished.
To finally create these diamond lattices, the Penn researchers used DNA-covered microspheres in two slightly different sizes.
“These spontaneously form colloidal crystals when incubated at the correct temperature, due to the DNA forming bridges between the particles,” Crocker said. “Under certain conditions, the crystals have a double diamond structure, two interpenetrating diamond lattices, each made up by one size or 'flavor' of particle.”
They then crosslinked these crystals together into a solid.
Crocker describes the achievement as good luck. The researchers hadn’t set out to create this diamond structure. They had been doing a “mix and pray” experiment: Wang was adjusting five material variables to explore the parameter space. To date, this has produced 11 different crystals, one of which was the surprising double diamond structure.
“Often times when something unexpected happens, it opens up a door to a new technological approach,” Sinno said. “There could be new physics as opposed to dusty old textbook physics.”
Now that they’ve cleared a significant hurdle on the path to creating PBMs, the researchers need to figure out how to switch out the materials for high index particles and selectively dissolve one species to leave them with one self-assembled diamond lattice of colloidal microspheres.
If able to successfully produce a PBM, the material would be like a “semi-conductor for light,” having unusual optical properties that don’t exist in any natural materials. Normal transparent materials have an index of refraction between 1.3 and 2.5. These PBMs could have a very high index of refraction, or even a negative index of refraction that refracts light backwards.
Such materials could be used to make lenses, cameras and microscopes with better performance, or possibly even “invisibility cloaks,” solid objects that would redirect all light rays around a central compartment, rendering objects there invisible.
Although the researchers have been able to reproduce this experimentally more than a dozen times, Sinno and Jenkins have been unable to reproduce the findings in simulation. It’s the only structure of the 11 crystals that Wang produced that they haven’t been able to replicate in simulation.
“This is the one structure we've found so far that we can't explain which is probably not unrelated to the fact that nobody predicted that you could form it with this system,” Sinno said. “There are several other papers we've had in the past that really show how powerful our approaches are in explaining everything. In a way, the fact that none of this worked adds evidence that something fundamentally different is taking place here.”
The researchers currently think that a different, unknown crystal grows and then transforms into the double diamond crystals, but this idea has proven difficult to confirm.
“You're used to writing papers when you understand something,” Crocker said. “So we had a dilemma. Normally when we find something we chew on it for a while, we do simulations and then when it all makes sense we write it up. In this case, we had to triple-check everything and then make a judgment call to say that this is an exciting discovery and other people beyond us can also work on this and think about and help us try to solve this mystery.”
This research was supported by a grant from the National Science Foundation.
RELATED JOURNAL ARTICLE