October 10, 2016
Katherine Kornei
UCLA physicists have shown that shining multicolored laser light on rubidium atoms causes them to lose energy and cool to nearly absolute zero. This result suggests that atoms fundamental to chemistry, such as hydrogen and carbon, could also be cooled using similar lasers, an outcome that would allow researchers to study the details of chemical reactions involved in medicine.
This research is published online today in the journal Physical Review X.
Physicists have known for several decades that shining laser light of a particular energy onto atoms can decrease their speeds and therefore energy. Small packets of light — photons — carry momentum with them, and this momentum is imparted to atoms as the photons are absorbed. By forcing atoms moving in one direction to absorb only photons traveling in the opposite direction, researchers can slow down the speeds of atoms from hundreds of meters per second to merely millimeters per second. This decrease in motion means that the atoms cool from room temperature to nearly absolute zero. Holding atoms at ultracold temperatures allows researchers to study their chemical reactions on the quantum mechanical level.
“Temperature is the main reason we don’t experience many things that require quantum mechanics in our daily lives,” said Wesley Campbell, a UCLA assistant professor of physics and astronomy, and co-author of the study. “At room temperature you don’t really know the details of what’s going on at the quantum scale. We can use these ultracold atoms to learn something about the chemistry that governs medicine and biology.”
However, designing the powerful lasers necessary for this cooling — lasers that emit light of only one specific color and therefore energy — is an engineering challenge. The wavelength of the laser light has to be controlled to roughly one part in 100 million, which is like measuring the width of the United States to a precision of five centimeters. Furthermore, cooling biologically important atoms like hydrogen, oxygen, and carbon requires laser light that’s in the very high-frequency ultraviolet part of the electromagnetic spectrum, and creating light this energetic is extremely difficult. In 1993, researchers in Italy successfully cooled hydrogen atoms using a laser, but the process was highly inefficient. “Nobody ever did it again,” Campbell said.
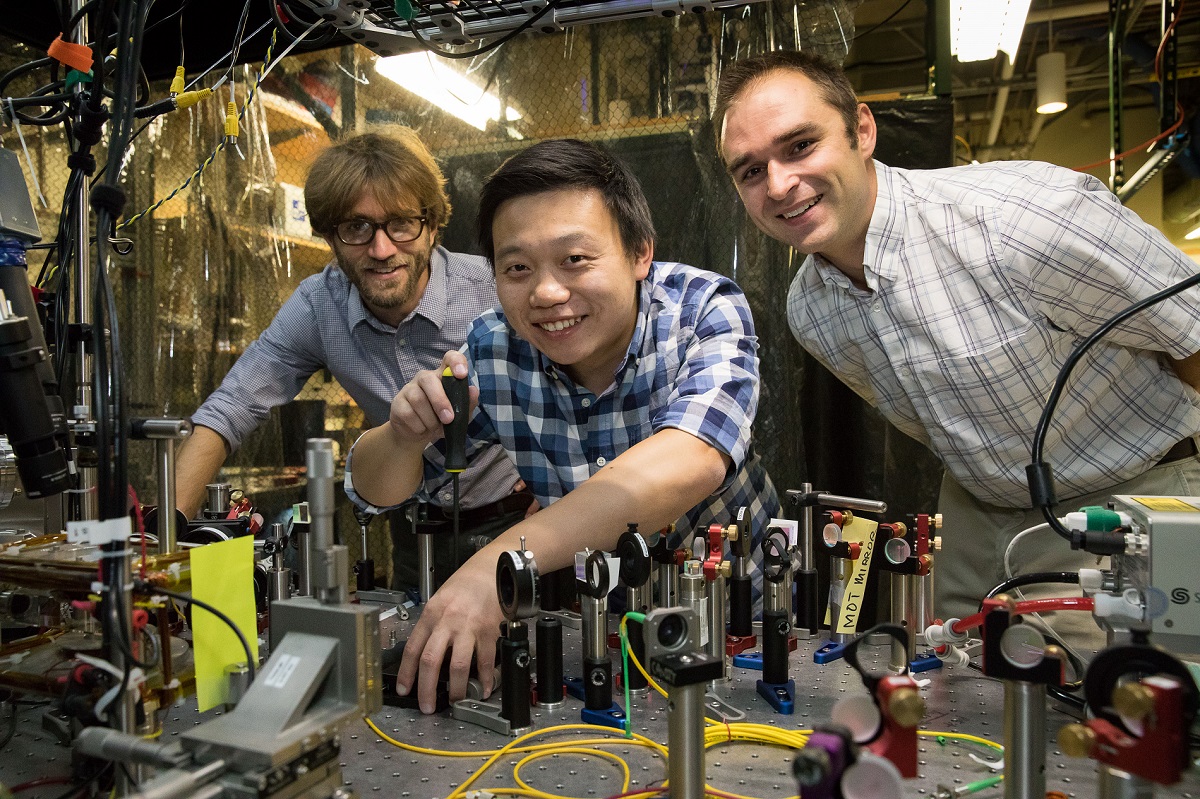
Campbell and his team demonstrated a proof of concept to show that biologically important atoms could be reliably cooled using lasers. The researchers faced two challenges: creating laser light of a precise energy and showing that very high-frequency ultraviolet radiation could be reliably generated.
The researchers adopted a surprising approach that involved using an atypical kind of laser. Instead of a laser that emits a single color of light — what most people think of when they envision a laser — Campbell and his group tested a laser that emitted a range of colors, all shades of red. “It’s a single laser, but it’s lots of colors,” said Xueping Long, a UCLA graduate student in Campbell’s laboratory and a co-author of the paper.
The researchers were initially worried that all of the slightly different red wavelengths of light would be detrimental to their experiment. “If you have light on at the wrong color, it typically heats things and causes problems,” said lead author Andrew Jayich, who was a UCLA postdoctoral researcher in Campbell’s laboratory when this research was conducted and is now an assistant professor of physics at UC Santa Barbara.
However, when the UCLA scientists pointed their multicolored laser beam toward roughly 10 million atoms, they successfully demonstrated that pairs of photons emitted by the laser could be simultaneously absorbed by atoms. When absorbed in tandem, these pairs of photons mimicked light with precisely the energy necessary to cool the atoms. “Two photons play the role of one photon with twice the energy,” Jayich said.
The researchers showed, for the first time, that pairs of photons could be used to reliably cool atoms. The final temperature of the atoms was 0.000057 degrees above absolute zero.
By combining red photons, the researchers demonstrated a method that can be used to create the energy of very high-frequency ultraviolet light, without the difficulties of actually generating such photons. “We’ve done something that sounds like it shouldn’t work at all,” Campbell said.
The scientists demonstrated their technique with rubidium, a heavy atom with 37 protons. “Rubidium is the easiest atom to work with; it’s nature’s gift to atomic physicists,” Campbell said. But he and his team are looking forward to how their technique can be adapted for use with more common elements such as hydrogen, carbon, oxygen and nitrogen.
“These atoms are what you and I are made of, what the planet is made of, and what stars are made of,” Campbell said. “We’re interested in getting access to these atoms that atomic physicists have basically given up on.”
The research was funded by the Air Force Office of Scientific Research Young Investigator Program grant and a National Science Foundation Career award (grant 1455357), and is part of the University of California Office of the President’s Research Catalyst Award (No. CA-15-327861).