Feb. 20, 2014
The Polymer Materials Unit of the National Institute of Materials Science (NIMS), in collaboration with Kyoto University, succeeded in the development of photocatalytic materials which can be activated by visible light, through the application of nanotechnology.
Research on the application of titanium dioxide as a photocatalyst has been carried out in many fields since it was discovered that this substance causes water splitting when exposed to ultraviolet light. For example, a titanium dioxide photocatalyst has been put into practical use for the decomposition of poisonous gas, etc. However, due to the scarcity of ultraviolet light contained in sunlight, this photocatalyst has not reached the stage where it can be used to cause the water splitting under sunlight and produce energy. In an attempt to develop photocatalytic materials which are more responsive to visible light, researchers have made efforts to improve a titanium dioxide photocatalyst or create a new photocatalyst made of materials other than titanium dioxide, but failed to develop a photocatalyst with desired efficiency.
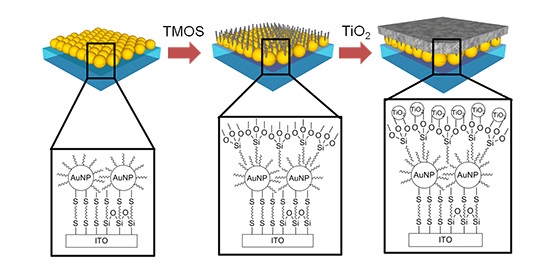
To overcome this challenge, the joint research team put titanium dioxide photocatalysts close to an array of metal nanoparticles, at a distance of about one nanometer (one-billionth of a meter), and used the nonlinearity of strong light caused within nanogaps between metal nanoparticles. By doing so, they were able to bring about photoexcitation equivalent to ultraviolet light with the use of visible light, which is the major component of sunlight. In fact, they examined the degradation reaction of a staining dye and found that the new photocatalyst reacted to visible light 6.5 times faster than the photocatalyst made only of titanium dioxide.
The research results will make it possible to use the new titanium dioxide photocatalyst as material in film electrodes of photoelectrode systems, or use it in combination with appropriate materials for reduction and thereby synthesize fuels and resources through hydrogen production resulting from decomposition of water or reduction of carbondioxide. The new photocatalyst will also be available for the decomposition and elimination of poisonous chemical substances. Accordingly, the application of photocatalysts, which have also been in a stage of practical use, is expected to be further expanded.
The research results have already been filed for a patent and will be published in the online version of Light: Science & Applications, on January 17, 2014, 17:00 (JST). This research was carried out as part of the research project titled “Space and Time Integration of Photochemical Reaction Intensified in the Near Field” (FY2010–FY2012; Leader: Kazushi Miki), applied for the program sponsored by the Ministry of Education, Culture, Sports, Science and Technology, “Grant-in-Aid for Scientific Research on Innovative Areas; Organic Synthesis Based on Reaction Integration: Development of New Methods and Creation of New Substances” (Leader: Junichi Yoshida, Professor, Graduate School of Engineering, Kyoto University) (http://www.sbchem.kyoto-u.ac.jp/syuuseki/index).