7 May 2015
Researchers from the UvA's Institute of Physics (IoP), together with international collaborators, have identified the way in which electrons form crystals within crystals. Their findings, described in two recent articles in the journal Nature Communications, could potentially allow researchers to manipulate the properties of materials containing these electron patterns and design materials for technological applications.
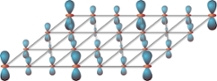
Crystals are solid materials in which the atoms are arranged into a regular, periodic structure. Well-known everyday examples include diamonds and rock salt. Whereas electrons generally float about freely within such atomic arrangements, in certain materials they spontaneously assemble into a rigid crystal structure of their own, unrelated to the surrounding atomic grid. In spite of the relative simplicity of the structures taken on by such crystals within crystals, the detailed drivers behind their occurrence in even the most well-studied materials have been clouded in mystery for decades. Several theories have been put forward, but none can explain the whole range of available experimental observations.
The shapes of electrons
To bridge this gap, two international research teams, both of which include UvA physicist Jasper van Wezel, conducted complementary experimental and theoretical studies on the origin of the electronic crystal within one representative material. 'Combining very different experimental techniques to study a single material allows us to really zoom in on its key features', Van Wezel explains. ‘Likewise, focusing on a single material means we can use knowledge about its specific properties in our calculations, which eventually allows us to reproduce, for the first time, all of the various experimentally observed properties within a single theoretical model.’
A crucial role in the model turns out to be played by the shapes electrons acquire within solid materials. Electrons in free space are generally described as point particles. But when they are embedded within solids, the electrons are actually better thought of as extended clouds. 'Some electron clouds are round like beach balls, others elongated like cigars, and some resemble the petals of a four-leaved clover', says Van Wezel. 'The way in which differently shaped electrons affect both each other and the atoms around them turns out to have a profound influence on the formation of the electronic crystal.'
Towards applications
By being able to predict and theoretically reproduce the formation of an electron crystal within one specific material, the researchers now hope to extrapolate their findings to other types of solids as well. This could result in ways of predicting the properties of as yet unstudied materials, and may perhaps even be used as a 'design tool' in the development of new material types.
Publication details
Felix Flicker and Jasper van Wezel: ‘Charge order from orbital-dependent coupling evidenced by NbSe2’, Nature Communications (7 May 2015). Doi: 10.1038/ncomms8034
U. Chatterjee, J. Zhao, M. Lavarone, R. Di Capua, J.P. Castellan, G. Karapetrov, C.D. Malliakas, M.G. Kanatzidis, H. Claus, J.P.C. Ruff, F. Weber, J. van Wezel, J.C. Campuzano, R. Osborn, M. Randeria, N. Trivedi, M.R. Norman and S. Rosenkranz: ‘Emergence of coherence in the charge-density wave state of 2H-NbSe2’, Nature Communications (19 January 2015). Doi:10.1038/ncomms7313.