May 17, 2013
By Andrew Myers
Among its many talents, silver is an antibiotic. Titanium dioxide is known to glom on to certain heavy metals and pollutants. Other materials do the same for salt.
In recent years, environmental engineers have sought to disinfect, depollute and desalinate contaminated water using nanoscale particles of these active materials. Engineers call them nanoscavengers. But the hitch from a technical standpoint is that it is nearly impossible to reclaim the nanoscavengers once they are in the water.
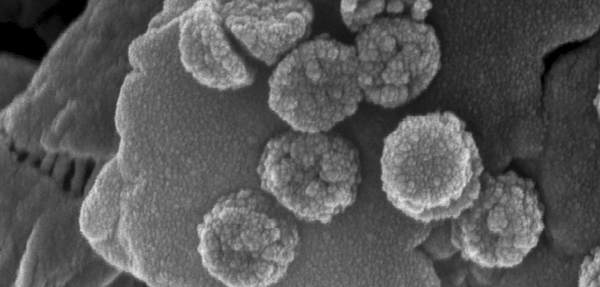
But now, an interdisciplinary team of engineers at Stanford University has developed a new type of nanoscavenger with a synthetic core that is ultraresponsive to magnetism, allowing the easy and efficient recovery of virtually every one of the nanoscale purifiers.
Their research appeared in a paper published online May 14 in the journal Nature Communications.
"In contaminated water, nanoscavengers float around, randomly bumping into bacteria and killing them or attaching themselves to molecular pollutants," said Shan Wang, the study's senior author and a professor of materials science and engineering and of electrical engineering. "When the contaminants are either stuck to the nanoscavenger or dead, the magnet is turned on and the particles vanish."
Ultraresponsive to magnetism
The use of magnetism to recover nanoscavengers is not new. There are commercial technologies today that have fashioned nanoscavengers with a core of magnetic iron oxide surrounded by an active material, but these ingenious methods are less than perfect. Iron oxide is not absolutely responsive to magnetism, and too many nanoscavengers remain in the water for it to be considered safe for human use.
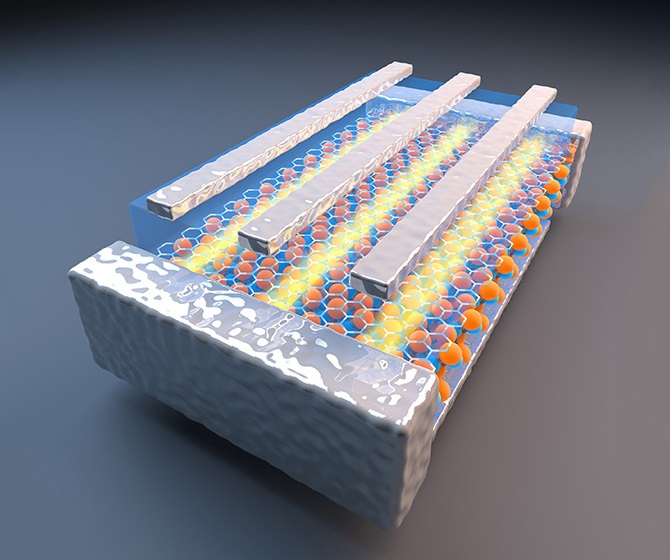
The Stanford advance replaces the iron oxide with a synthetic material. The Stanford core is, in reality, not a single material, but a disk of several layers – magnetic outer layers of the synthetic material sandwiched on either side of a titanium center, but with a twist.
"The magnetic moments of the two outer layers are opposed. The direction of the magnetic force in the top layer and the bottom layer point in opposite directions, effectively canceling the magnetic properties of the material," said Mingliang Zhang, a doctoral candidate in materials science and engineering and co-first author of the study.
In their natural state, the new nanoscavengers are not magnetic and would not be attracted to another magnetic material. When the composite discs are exposed to a strong magnetic field, however, the opposing directions of magnetic force of the two layers turn into alignment, not only making the material magnetic but compounding the magnetic effect.
Side-by-side tests
In doing so, the nanoscavengers become ultraresponsive to magnetism, far more so than the base iron oxide used in today's technologies. The Stanford team has dubbed its advance with the oxymoronic name "synthetic antiferromagnetic cores." The prefix anti- in this case means in opposite direction, not non-magnetic.
With a successful core created, the researchers then cap it with silver or titanium dioxide or another reactive material, depending upon the contaminant they are targeting.
In live tests using synthetic-core, silver-capped nanoscavengers immersed in water tainted with E. coli bacteria, using a silver dosage of just 17 parts per million, the Stanford team was able to kill 99.9 percent of the bacteria in just 20 minutes. Better yet, they removed virtually all of the nanoscavengers in just five minutes of exposure to a permanent magnet.
Side-by-side tests of the effectiveness of the same magnet on iron oxide core nanoscavengers show a quick collection of about 20 percent of the nanoscavengers in the same five minutes, but then the effect plateaus. By minute 20, nearly 8 in 10 iron oxide core nanoscavengers remain in the water.
The one-pot solution
Having demonstrated a working prototype, the team is now building various iterations of their nanoscavengers with different reactive exteriors to target specific pollutants, as well as a new class of slightly larger nanoscavengers that might bear discrete bands of several different reactants.
"Our hope is to one day create a 'one-pot solution' that tackles water afflicted by a diverse mixture of contaminants. A purification technology like that could be very useful in recycling water in developing nations, or in arid climates like the American West, where water quality and quantity are of critical importance," added Xing Xie, a doctoral candidate in civil and environmental engineering and co-first author of the paper.
Contributors to this study include Craig Criddle, professor of civil and environmental engineering; Yi Cui, associate professor of materials science and engineering; and Mary Tang, senior research engineer.
Andrew Myers is associate director of communication for the Stanford University School of Engineering.