25 Jul 2013
In a perspective review written for Nature, Sir Andre and Dr Irina Grigorieva, from The University of Manchester, discuss how layered materials can be split into isolated atomic planes and then reassembled back in an intelligently-chosen sequence to create a new kind of materials and structures that do not exist in nature.
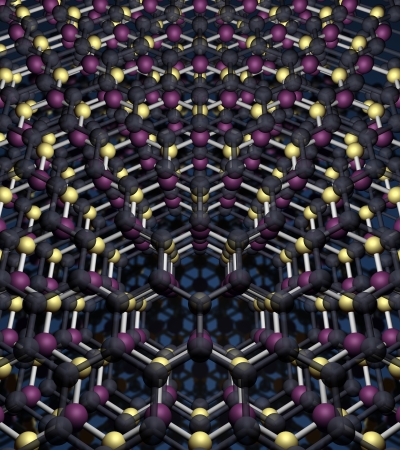
The authors point out that in addition to graphene there are many other one atom or one molecule thick crystals. These include monolayers of boron nitride (otherwise known as ‘white graphene’) and molybdenum disulphide which are already well characterized and attracted significant attention from academia and industry. The search for more atomically thin crystals is growing rapidly.
The graphene-like materials promise a range of their own applications but, in isolation, they are unlikely to offer the same remarkable properties as graphene itself – the world’s thinnest, strongest and most conductive material.
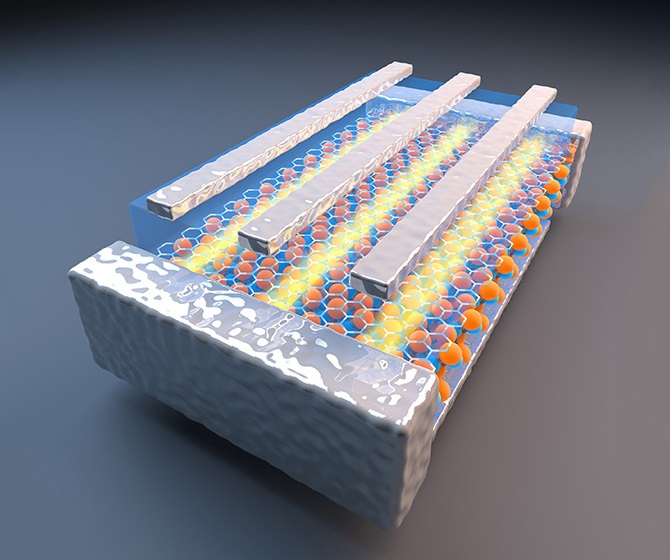
The most exciting development suggested, proven and now reviewed by University of Manchester researchers was creating ‘atomic Lego’; stacking these atomically-thin materials in heterostructures and artificial materials so that the resulting properties can be controlled and manipulated. Such materials made with a single plane precision could not be made by any previously-known technique.
The combinational structures have the ability to go beyond graphene’s long list of superlatives and create applications and devices which, until now, have existed only in science fiction. For example, the atomic scale Lego has already been used to improve the electronic quality of graphene and make graphene transistors with high on-off ratios suitable for integrated circuits.
Currently, sophisticated multilayer structures are put together in university labs in a matter of days by manual assembly under an optical microscope. This is sufficient to scout for the most promising combinations aiming at a particular application. In future, this assembly can be made on an automated industrial basis by using machinery similar to those which currently produce graphene rolls hundreds metres long.
Sir Andre said: “I believe this new research field is going to be as big as graphene itself. It is already clear that graphene combined with other atomically-thin materials shows properties better or different from its own.
“Because of the amount of possibilities of combining these graphene-like materials together in heterostructures is practically unlimited, there must be new materials with unique properties no-one has even dreamed of as yet. We are spoiled for choice.”
Dr Grigorieva added: “With so many graphene-like crystals out there, we are limited only by our imagination how to combine them. That is a very exciting time to be a materials scientist and explore this terra incognito of materials that you can literally design and then make layer by layer yourself.”
The paper, Van der Waals heterostructures, by A. K. Geim and I. V. Grigorieva, is available on request from the Press Office.
More information about graphene can be found at www.graphene.manchester.ac.uk