July 02, 2014
Scientists don't fully understand how ‘plastic' solar panels work, which complicates the improvement of their cost efficiency, thereby blocking the wider use of the technology. However, researchers at the University of Montreal, the Science and Technology Facilities Council, Imperial College London and the University of Cyprus have determined how light beams excite the chemicals in solar panels, enabling them to produce charge. “Our findings are of key importance for a fundamental mechanistic understanding, with molecular detail, of all solar conversion systems – we have made great progress towards reaching a ‘holy grail' that has been actively sought for several decades,” said the study's first author, Françoise Provencher of the University of Montreal. The findings were published today in Nature Communications.
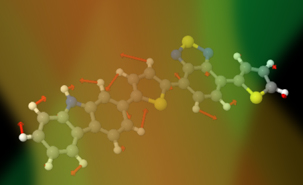
The researchers have been investigating the fundamental beginnings of the reactions that take place that underpin solar energy conversion devices, studying the new brand of photovoltaic diodes that are based on blends of polymeric semiconductors and fullerene derivatives. Polymers are large molecules made up of many smaller molecules of the same kind – consisting of so-called ‘organic' building blocks because they are composed of atoms that also compose molecules for life (carbon, nitrogen, sulphur). A fullerene is a molecule in the shape of a football, made of carbon. “In these and other devices, the absorption of light fuels the formation of an electron and a positive charged species. To ultimately provide electricity, these two attractive species must separate and the electron must move away. If the electron is not able to move away fast enough then the positive and negative charges simple recombine and effectively nothing changes. The overall efficiency of solar devices compares how much recombines and how much separates,” explained Sophia Hayes of the University of Cyprus, last author of the study.
Two major findings resulted from the team's work. “We used femtosecond stimulated Raman spectroscopy,” explained Tony Parker of the Science and Technology Facilities Council's Central Laser Facility. “Femtosecond stimulated Raman spectroscopy is an advanced ultrafast laser technique that provides details on how chemical bonds change during extremely fast chemical reactions. The laser provides information on the vibration of the molecules as they interact with the pulses of laser light.” Extremely complicated calculations on these vibrations enabled the scientists to ascertain how the molecules were evolving. Firstly, they found that after the electron moves away from the positive centre, the rapid molecular rearrangement must be prompt and resemble the final products within around 300 femtoseconds (0.0000000000003 s). A femtosecond is a quadrillionth of a second – a femtosecond is to a second as a second is to 3.7 million years. This promptness and speed enhances and helps maintain charge separation. Secondly, the researchers noted that any ongoing relaxation and molecular reorganisation processes following this initial charge separation, as visualised using the FSRS method, should be extremely small. “Our findings open avenues for future research into understanding the differences between material systems that actually produce efficient solar cells and systems that should as efficient but in fact do not perform as well. A greater understanding of what works and what doesn't will obviously enable better solar panels to be designed in the future,” said the University of Montreal's Carlos Silva, who was senior author of the study.
About the study:
The article “Direct observation of ultrafast long-range charge separation at polymer–fullerene heterojunctions,” was published in Nature Communications on July 1, 2014. Françoise Provencher is Carlos Silva's student at the University of Montreal's Department of Physics. Both are affiliated with the Regroupement québécois sur les matériaux de pointe. Silva is also a visiting professor at Imperial College London. Sophia Hayes is affiliated with the Department of Chemistry at the University of Cyprus. The instrumental scientists of the Science and Technology Facilities Council (UK) Anthony W. Parker, Gregory M. Greetham, Michael Towrie, set-up the laser system and experiments. Nicolas Bérubé, Christoph Hellmann, Michel Coté, and Natalie Stingelin also contributed to the research. The researchers received funding from the Natural Sciences and Engineering Research Council of Canada, the Canada Research Chair in Organic Semiconductor Materials, the Royal Society, the Leverhulme Trust, LASERLAB-EUROPE (grant agreement no. 284464, EC's Seventh Framework Programme), the UK Engineering and Physical Sciences Research Council (EP/G060738/1 grant), the European Research Council (ERC) Starting Independent Research Fellowship (grant agreement No. 279587) and King Abdullah University of Science and Technology. The Science and Technology Facilities Council supported access to the FSRS facilities beyond the EU support typically offered. The University of Montreal is known as Université de Montréal.