May 06, 2015
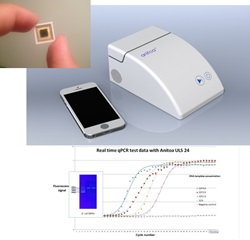
Anitoa Systems, (“Anitoa”), a Palo Alto startup established in 2011, partnering with Zhejiang University of China, has successfully demonstrated a handheld real time quantitative-polymerase-chain-reaction system (qPCR) using Anitoa's ultra-low-light CMOS bio-optical sensor. Anitoa’s handheld qPCR is shown to be capable of detecting several types of pathogen DNA and RNA’s, including hepatitis B, C, and E. Coli. This qPCR system has achieved detection limit of 4 copies per sample and over 9 orders of magnitude dynamic range. A key component of this handheld qPCR is the CMOS bio-optical sensor chip produced by Anitoa. It formed a single-chip fluorescent imaging system, tightly integrated with a miniature thermal cycler, to perform real time imaging of multiple PCR reaction sites simultaneously without the need for a scanning mechanism commonly used by qPCR systems today.
Released in September 2014, Anitoa’s ultra-low light CMOS bio-optical sensor is the first commercially available CMOS imager sensor that has the needed sensitivity to replace photon multiplier tubes (PMT) and cooled-CCDs in a wide range of medical and scientific instruments, such as a qPCR instrument. The ultra-low light sensitivity (3e-6 lux) of Anitoa’s CMOS sensor is crucial for achieving good signal-to-noise ratio (SNR) in imaging molecular interactions based on fluorescent or chemiluminescence signaling principle. “We are very pleased to see the test results coming back from partner hospital showing the effectiveness of detecting infectious pathogens. This not only validates the CMOS bio-optical sensor’s ultra-low-light sensitivity, but also its applicability to real world disease diagnostics”, said Anitoa CEO Dr. Zhimin Ding.
With its bio-optical sensor innovation, Anitoa is developing a low cost, portable, yet high performance qPCR-based molecular diagnostics platform as a globally-affordable tool to help fight infectious diseases worldwide. By using the integrated and low-power CMOS bio-optical chip instead of the bulkier and more expensive CCD (Charge Coupled Device) or PMT (Photon Multiplier Tube) devices for fluorescent imaging, system designers can now achieve significant space and cost savings by doing away with those auxiliary components found in a PMT or CCD based system necessary for signal acquisition, high voltage supply and regulation, and heat ventilation. In addition, multiple CMOS bio-optical sensors can be deployed to provide wavelength multiplexing imaging capability. This capability allows sensing multiple reaction sites with very flexible format, and is thus optimal when combined with microfluidics to provide ability to detect a large number DNA targets, and integrate with upstream processes such as sample preparation. Such integrated system can minimize hands-on time and the possibility of contamination when used in diagnostics of infectious disease.
Availability
A CMOS-based qPCR reference design that includes a thermal cycler and matching fluorescent imaging subsystem is available from Anitoa for selected OEM customers. Please send your inquiry to info(AT)anitoa.com. Anitoa’s ultra-low-light CMOS bio-optical sensor can also be integrated into other platforms and applied to other applications requiring sensitive imaging of fluorescence or chemiluminescence-labeled molecular targets. A development kit of Anitoa's ultra low-light CMOS bio-optical sensor is commercially available now. The information is can be found at http://www.anitoa.com/docs/solution_kit_brief.pdf.
About Anitoa
Anitoa Systems, established in 2011 with headquarters at Palo Alto, California, develops highly-integrated CMOS system-on-chip (SoC) products for a new generation of medical and scientific instruments. Anitoa’s molecular sensor technology, consisting of a CMOS Bio-Optical Sensor and proprietary Intelligent Dark-current Management algorithm, enables compact and field-portable molecular diagnostics platforms targeting near-patient tests of infectious diseases.
Anitoa partners with Zhejiang Nano-systems Institute (ZCNI), and the First Affiliated Hospital of Zhejiang University in China for clinical application validation and field deployment.