July 04, 2013
The radioactive element astatine, the name of which is derived from the Greek word for 'instability,' is so rare on earth that it has not yet been investigated to any greater extent and, as a consequence, very little is known about it. Using artificially generated astatine, the Mainz-based physicist Sebastian Rothe has now managed for the first time to experimentally explore one of its fundamental parameters, the ionization potential, and thus determine one of the most important properties of the rare element. The ionization potential is the binding energy, i.e., the amount of energy required to remove an electron from an atom's outer shell. It determines the entire chemical binding characteristics of that element. The measurements were undertaken at the laboratory of the CERN European Organization for Nuclear Research near Geneva using special lasers developed by the LARISSA working group at the Institute of Physics at Johannes Gutenberg University Mainz (JGU). The online journal Nature Communications recently published the findings.
Astatine is the rarest naturally occurring element on earth. The earth's mantel is estimated to contain only 0.07 grams. Together with fluorine, chlorine, and iodine, it is a member of the halogen group, and is formed as a result of the natural decay of uranium. Nuclear physicists now know of more than 20 isotopes which are all extremely short-lived and decay with a half-life of no more than eight hours. Alpha rays are emitted during decay, making the element particularly interesting for targeted cancer therapy thanks to its short lifespan. "Astatine is the only halogen we have known absolutely nothing about to date", explained Professor Klaus Wendt, head of the LARISSA working group at the Institute of Physics at Johannes Gutenberg University Mainz (JGU). A doctoral candidate and member of this work group, Sebastian Rothe, investigated the ionization potential of astatine using laser spectroscopy and determined it had a value of 9.31751 electron volts (eV). The measurements were conducted at CERN in Geneva and were extrapolated and confirmed at the Canadian research center for particle and nuclear physics TRIUMF in Vancouver in Canada.

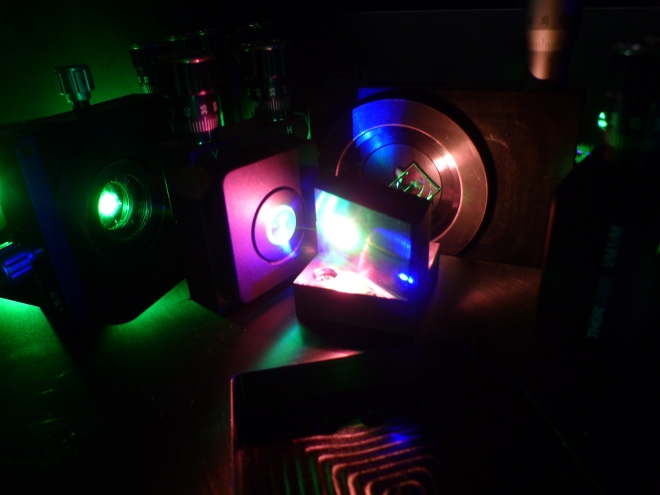
Close-up of the Mainz laser system
photo: Pascal Naubereit
LARISSA is an acronym for 'Laser Resonance Ionization for Spectroscopy in Selective Applications'. The technique is based on work originally conducted by Mainz physicist Professor Ernst Otten more than 30 years ago using the isotope mass separator ISOLDE at CERN. It is now the technique of choice employed at almost all large-scale research facilities throughout the world to produce and examine exotic radioisotopes and is commonly used applying the Mainz laser system. It involves the use of laser light for the gradual optical excitation of a valence electron of a selected atomic species until the point of ionization.
"Astatine is the last naturally occurring element whose ionization potential had yet to be determined experimentally," stated Rothe. The binding energy of the electrons in its outermost shell determines what chemical reactions astatine will undergo and thus the stability of its chemical bonds. It is believed that the astatine isotope 211 may have a major pharmaceutical potential. It is an exceptional candidate for use in cancer therapy because of its decay profile, the aggressiveness of its alpha radiation, and the limited range of its radiation. It is also a member of the halogen family, which can be readily introduced into the human body to be attached directly to cancer cells.
Publication
Sebastian Rothe et al., Measurement of the first ionization potential of astatine by laser ionization spectroscopy, Nature Communications, May 2013
DOI: 10.1038/ncomms2819