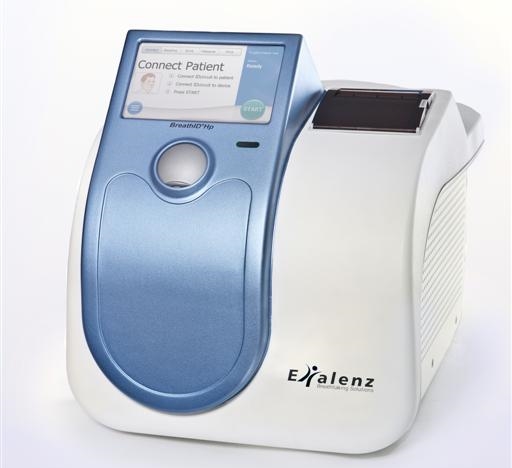
Scheduled for worldwide launch in July, Exalenz's advanced BreathID System is expected to accelerate revenues and improve gross margin significantly
Mr. Larry Cohen, CEO of Exalenz:
"Following years of development and successful completion of trials, which included hundreds of patients in the U.S. and in Israel, Exalenz launches a new system with unmatched performance. Combined with a recently established marketing and sales program, we expect a significant improvement in company performance in the third quarter."
May 26, 2013 - Exalenz Bioscience, -, a developer and marketer of unique medical devices for the diagnosis and management of digestive system and liver conditions, today proudly announced the receipt of clearance from the U.S. Food and Drug Administration (FDA) for the marketing of the Company's newly developed BreathID System in the United States. The system was developed over several years and approximately 330 patients have participated in clinical trials in the U.S. and in Israel. FDA approval was granted several weeks after the Company had received CE approval for marketing the system in Europe.
The BreathID Hp device for Helicobacter pylori (H. pylori) detection is an advanced, new-generation of the original BreathID device that was developed by the Company. The BreathID device sold in the U.S. has proven high clinical efficacy in applications for detection of H. pylori bacterium.
The new BreathID Hp device is designated, in the first phase, for the purpose of H.pylori testing, yet it is designed to serve as a platform for additional applications in the future. The new system will replace the original device, which was developed over a decade ago. Exalenz intends to begin the worldwide marketing and sales of BreathID Hp in July. The new system, which is by far more advanced than existing systems by competition, delivers better performance than any other device marketed worldwide. Thanks to the advanced technologies that were applied in its development, the system offers greater ease-of-use, and a small foot print as well as additional features such as electronic medical record (EMR) ready capability. The cost to manufacture the system will also be significantly less than the previous system. The Company expects that the cost reductions will result in higher gross margin in the next quarter.
Exalenz estimates that the high added value offered to physicians and medical centers by the new BreathID Hp device will further improve company sales and will contribute to accelerating sales in the U.S. market and to improving gross margins significantly.
Marketing of the new system in the U.S. will be executed by the new marketing and sales array that is currently established by the Company's new CEO, Mr. Larry Cohen, who, prior to this position, had directed sales volumes of billions of U.S. dollars for diagnostic companies. The establishment of the new marketing system and the preparations for the launch of the new system constituted the majority of the Company's activities in the U.S. in the second quarter, during which the Company had ceased to focus on selling previous generation devices based on its expectation of U.S. approval.
Mr. Larry Cohen, CEO of Exalenz, said today that "we are very proud to receive FDA approval for the BreathID Hp device. This device embodies the proven, high capabilities of the BreathID technology, which was developed by the Company, and is currently sold successfully as an H. pylori testing application in over 200 medical centers in the U.S."
"We are confident that the new system will assist us to accelerate Exalenz's sales in the U.S. and is likely to become a significant strategic milestone in the Company's business development. The satisfaction among medical centers in the U.S. regarding the use of the BreathID system alongside the new system's improved functional capabilities reassures us that we can increase the demand for this unique solution, and this will constitute a crossroad in the Company's development. The significant change in the gross margin will enable us to proceed towards achieving operating profit in the foreseeable future. The new system demonstrates Exalenz's aspiration and commitment to innovation, creativity and excellence that meet customer's needs."
Exalenz, under the direction of Mr. Larry Cohen, is working to establish a marketing and sales array to allow a greater penetration rate of the systems to the U.S. market. As of the end of the first quarter of 2013, the Company has been supplying approximately 216 medical centers in the U.S., which purchased the BreathID system.
About Exalenz Bioscience
Exalenz Bioscience develops medical equipment for breath testing for the purposes of diagnosis and management of digestive system and liver conditions. The company sells the BreathID® System together with required disposable test kits.
The BreathID® device developed by the Company has been proven effective for clinical use in identifying the presence of the H. pylori bacteria and is presently undergoing clinical tests for use in the field of liver function. Exalenz holds regulatory approvals in Europe and the U.S. for some of the applications, and the Company is currently in process of applying for approvals for additional applications.
For more information: