26 June 2013
For the first time, a research team from the CEA1, Collège de France, CNRS and Université Joseph Fourier in Grenoble has developed an efficient process for in vitro activation of an enzyme, hydrogenase, which is found in microorganisms that use hydrogen as a source of energy. This was made possible by combining approaches from biomimetic and protein chemistry. The results will lead to use of the wide variety of hydrogenase enzymes found in nature, and even the possibility of ‘invented’ artificial enzymes over the long term, which may potentially serve as catalysts for fuel cells or the production of hydrogen from sources of renewable energy.
The results were published online in Nature on 26 June 2013.
The production of hydrogen through water electrolysis and its later use as fuel, for example in fuel cells, offers interesting possibilities in the area of energy storage, particularly for renewable energy. Such techniques, which are both high performance and promising, nevertheless require the use of catalysts containing precious metals, such as platinum, which are rare and expensive. Alternative solutions must be sought.
Some microorganisms, particularly microalgae, can produce hydrogen or use it as a source of energy to sustain their metabolism. For catalysts, they use metalloenzymes composed of metals found in abundance, such as iron. These metalloenzymes with remarkable catalytic properties are known as hydrogenases. Today, they represent a natural alternative to platinum for preparing increasingly efficient green electrolyzers and fuel cells. The active sites of these enzymes are nevertheless complex and their biosynthesis requires specific biological machinery, still poorly known and described, that works efficiently only in cellulo.
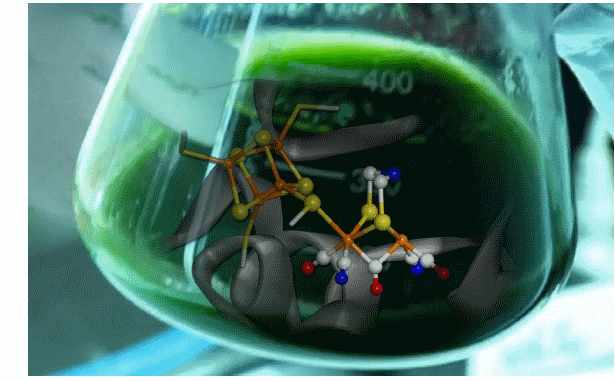
Researchers from the CEA, Collège de France, CNRS and Université Joseph Fourier in Grenoble2, members of the ARCANE laboratory of excellence3, have just developed a reagent that can transform, in vitro and very efficiently, an inactive hydrogenase4 into a completely active hydrogenase. This new reagent, composed of a synthetic biomimetic complex (a small cluster of analogous iron from the active site) and a stabilizing protein, can react with inactive hydrogenase by transferring the synthetic biomimetic part. The structure of the latter is sufficiently similar to the natural active site that it provides the reconstituted enzyme its natural catalytic energy. To obtain this result, the researchers used a multidisciplinary approach based on organometallic, biomimetic and protein chemistry and spectroscopy.
‘Artificial’ activation of hydrogenases offers promising possibilities both for basic research and applications. These results increase understanding of the impact of the protein environment on the reactivity of the enzyme’s active site. The new data will facilitate exploration of natural hydrogenases for seeking the most efficient and stable enzyme for technological applications.
This process may eventually lead to the ‘invention’ of new enzymes and artificial hydrogenases through the synthesis of analogs of various active sites. This would mean any number of potential new catalysts for future fuel cells and the production of hydrogen from renewable energy.
Reference:
“Biomimetic assembly and activation of [FeFe]-hydrogenases"
G. Berggren, A. Adamska, C. Lambertz, T. Simmons, J. Esselborn, M. Atta, S. Gambarelli, JM Mouesca, E. Reijerse, W. Lubitz, T. Happe, V.Artero, M. Fontecave.
1 Team composed of the Chemistry and Biology of Metals Laboratory (LCBM, a joint CEA/CNRS/Université Joseph Fourier unit) ‐ Institute of Life Sciences Research and Technologies (IRTSV) – Institute for nanoscience and cryogenics (INAC).
2This work is in collaboration with the Max Planck Institute for Chemical Energy Conversion in Mülheim and the Ruhr‐Universät Bochum (RUB) in Germany.
3 ARCANE is a laboratory of excellence in the area of sustainable chemistry for the health and renewable energy sectors that brings together all authors of this publication.
4 Hydrogenase that lacks the active site is known as apo‐hydrogenase.