2015-02-04
It is known that hydrogen is a clean and transportable carrier for renewable energy. Thus water splitting to produce hydrogen by harvesting solar energy has become a highly important technology for clean energy. Semiconductor catalysts play important roles in the photocatalytic water splitting, such as light harvesting and reaction barrier reduction. Silicon as the semiconductor material with high natural abundance and fabrication processability has been predicted usable for photocatalytic water splitting. Most recently, the research group led by Prof. XIONG Yujie elucidated the nature of photocatalytic “water splitting” on silicon nanowires for the first time, and provided a promising approach to performance improvement of hydrogen production. This work has been published online in Angewandte Chemie entitled “The Nature of Photocatalytic ‘Water Splitting’ on Silicon Nanowires” (Angew. Chem. Int. Ed.
DOI: 10.1002/anie.201411200;http://onlinelibrary.wiley.com/doi/10.1002/anie.201411200/abstract) and selected as the Hot Paper of Angewandte Chemie.
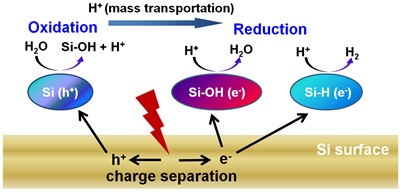
In this work, the research team creatively combined micro- and nanofabrication techniques (top-down) with wet-chemical method (bottom-up) so that the types and amounts of dangling bonds could be selectively controlled on the surface of silicon nanowire arrays. Based on the correlation of hydrogen production efficiency and average carrier lifetime with surface dangling bonds, they have been able to reveal the key roles of dangling bonds in photocatalytic applications. Meanwhile, the researchers found that the ratio of produced H2 to O2 is far above 2 – the number anticipated for water splitting, and as such, proposed that this reaction should follow a different mechanism from conventional water splitting. The theoretical simulations by Prof. Jun Jiang not only validated the expected contributions of dangling bonds to charge separation, but also obtained the reaction barriers on the surfaces with different dangle bonds. Based on this series of findings, the research team for the first time elucidated the nature of photocatalytic “water splitting” on silicon materials.
Upon acquiring the working mechanism, the researchers further developed surface modification methods based on semiconductor industry techniques, and provided a convenient approach to modulate the states of dangling bonds on nanowire surface for tunable photocatalytic hydrogen production performance. It is anticipated that the impact of surface chemistry on solar energy conversion is not limited to photocatalytic water splitting and can be applied to other applications including photoelectrochemical devices and pollution remedy. This work thus opens a new window to rationally designing photoactive materials for various reaction systems.
This work was supported by 973 Program, NSFC, Specialized Research Fund for the Doctoral Program of Higher Education, Recruitment Program of Global Experts, CAS Hundred Talent Program, and Fundamental Research Funds for the Central Universities.
(XIONG Yujie, HFNL)