13 April 2017
Physicists image individual molecules by watching them absorb light Molecules are extremely hard to see in visible light, especially without using fluorescence. Leiden physicists have now made their optical technique sensitive enough to image the molecules of their interest in all sizes. Publication in Nanoletters.
What do ships, bats and torpedoes have in common? They navigate by emitting sound waves and registering listening where those get absorbed or reflected. Humans do the same with light waves, except that they rely on external sources like the sun for the original emission. However when looking at something as small as a single molecule this becomes problematic, as light waves, not to mention sound waves, are bigger than the object itself.
Two light beams
In 2010, Leiden physicist Michel Orrit became the first to optically image large single organic molecules at room temperature without using fluorescence. Now, he and his group have made their technique much more sensitive, enabling them to image their subjects objects of interest—light-sensitive conducting polymer molecules—of all sizes. Just like bats, they control their own source of waves and use varying frequencies. Their first light beam has a specific color which only the targeted molecules can absorb. This causes them to heat up a bit. And because of thermal expansion this changes the refractive index of the surrounding liquid, so that a second beam will be scattered differently at the exact places where the molecules of interest are hiding.
Critical fluid
Still, this is easier said than done. Conducting polymers are quickly damaged by light, so scientists have to be extremely careful to only use very low intensities. But those are not nearly strong enough for the absorb-and-heat technique in regular liquids. Fortunately, so-called critical fluids are exceedingly sensitive to temperature changes within a small temperature range. In that regime, even the slightest heating power will alter the liquid’s refractive index by a large amount. So Orrit and his group used critical fluids and made sure temperature and pressure were precisely set during their experiment.
Locate them all
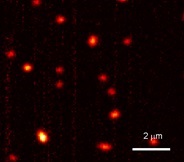
‘Until now we could only image the largest polymer molecules through absorption,’ says Orrit. ‘But because of our sensitivity enhancement, we can locate all of them. And this also gives us information on the brightness of each molecule. That is very important if you want to optimize their optoelectronic applications.’
Publication