September 13, 2017
Ali Sundermier
Glass is everywhere. Whether someone is gazing out a window or scrolling through a smartphone, odds are that there is a layer of glass between them and whatever it is they’re looking at.
Despite being around for at least 5,000 years, there is still a lot that is unknown about this material, such as how certain glasses form and how they achieve certain properties. Better understanding of this could lead to innovations in technology, such as scratch-free coatings and glass with different mechanical properties.
Over the past few years, researchers at the University of Pennsylvania have been looking at properties of stable glasses, closely packed forms of glasses which are produced by depositing molecules from a vapor phase onto a cold substrate.
“There have been a lot of questions,” said Zahra Fakhraai, an associate professor of chemistry in Penn’s School of Arts & Sciences, “about whether this is analogous of the same amorphous state of naturally aged glasses such as amber, which are formed by just cooling a liquid and aging it for many, many years.”
In order to answer these questions, Fahkraai and Ph.D. student Tianyi Liu collaborated with chemistry professor Patrick Walsh who designed and synthesized a new special molecule that is perfectly round with a spherical shape. According to Fakhraai, these unique molecules can never align themselves with any substrate as they are deposited. Because of this, the researchers expected the glasses to be amorphous and isotropic, meaning that their constituent particles, whether they are atoms, colloids or grains, are arranged in a way that has no overarching pattern or order.
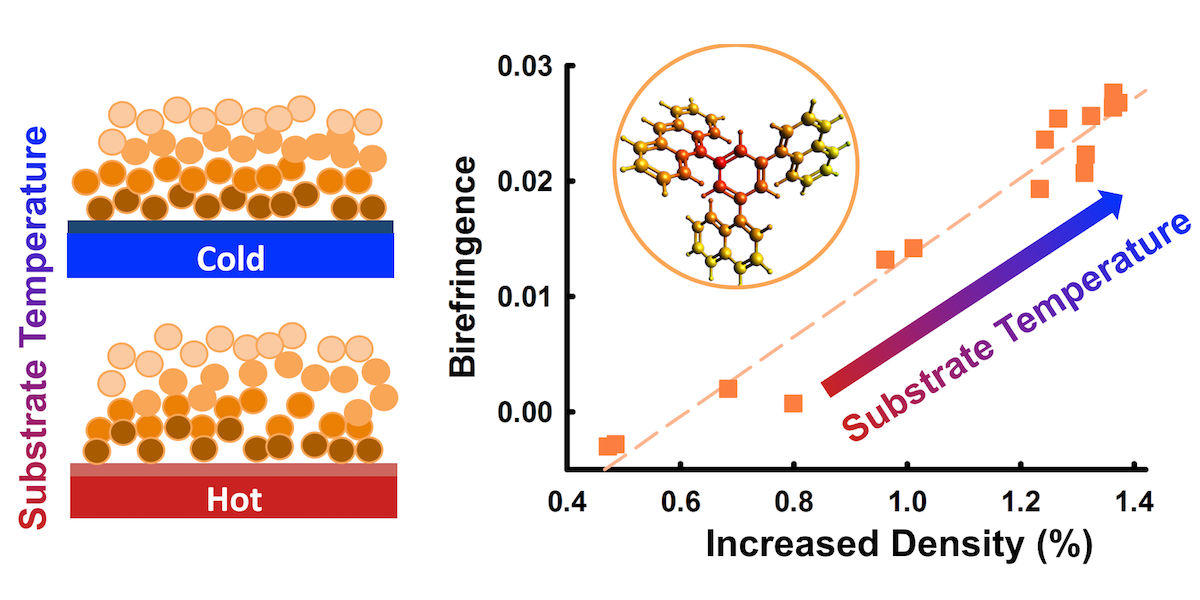
Surprisingly, the researchers noticed that these stable glasses are birefringent, meaning the index of refraction of light is different in directions parallel and normal to the substrate, which wouldn’t be expected in a round material. Their results were published in Physical Review Letters.
With birefringence, light shined in one direction will break differently than light shined from a different direction. This effect is often harnessed in liquid crystal displays: changing the orientation of the material causes light to interact differently with it, producing optical effects. In most deposited glasses, this is a result of molecules aligning in a particular direction as they condensate from the vapor phase into a deep glassy state.
The birefringence patterns of the stable glasses were strange, Fakhraai said, as the researchers did not expect any orientation of these round molecules in the material.
After teaming up with physics professor James Kikkawa and Ph.D. student Annemarie Exarhos, who did photoluminescence experiments to look at the orientation of the molecules, and chemistry professor Joseph Subotnik and Ph.D. student Ethan Alguire, who helped with the simulations aimed at looking at the crystal structure and calculating the index of refraction of the crystal which allowed them to work out the math of the degree of birefringence or ordering in the amorphous state, the researchers confirmed their hunch that there was no orientation in the material.
Despite measuring zero order in the glass, the scientists still saw an amount of birefringence analogous to having up to 30 percent of the molecules perfectly ordered. Through their experiments, they found that this is due to the layer-by-layer nature of the deposition that allows molecules to pack more tightly in the direction normal to the surface during the deposition. The denser the glass, the higher the value of birefringence. This process can be controlled by changing the substrate temperature that controls the degree of densification.
“We were able to show that this is a unique kind of order that is emergent from the process,” Fakhraai said. “This is a new sort of packing that's very unique because you don't have any orientation, but you can still manipulate the molecular distances on average and still have a random but birefringent packing overall. And so this teaches us a lot about the process of how you can actually access these lower state phases but also provides a way of engineering optical properties without necessarily inducing an order or structure in the material.”
Since the stressors are distributed differently in and out of plane, these glasses could have different mechanical properties, which may be useful in coatings and technology. It may be possible to manipulate the orientation of a glass or its layering to give it certain properties, such as anti-scratch coatings.
“We expect that if we were to indent the glass surface with something,” Fakhraai said, “it would have different toughness versus indenting it on the side. This could change its fracture patterns or hardness or elastic properties. I think understanding how shape, orientation and packing could affect the mechanics of these coatings is one of the places where interesting applications could emerge.”
According to Fakhraai, one of the most exciting pieces of this research is the fundamental aspect of now being able to show that there can be amorphous phases that are high density. She hopes she and other researchers can apply their understanding from studying these systems to what would happen in highly aged glass.
“This tells us that we can actually make glasses that have packings that would be relevant to very well-aged glass,” Fakhraai said. “This opens up the possibility of better fundamentally understanding the process by which we can make stable glasses.”
This research was funded by National Science Foundation grants DMR-11-20901, DMR-1206270, CHE-1152488 and DMREF-1628407.