February 25, 2016
AMHERST, Mass. – Chemists and polymer scientists collaborating at the University of Massachusetts Amherst report in Nature Communications this week that they have for the first time identified an unexpected property in an organic semiconductor molecule that could lead to more efficient and cost-effective materials for use in cell phone and laptop displays, for example, and in opto-electronic devices such as lasers, light-emitting diodes and fiber optic communications.
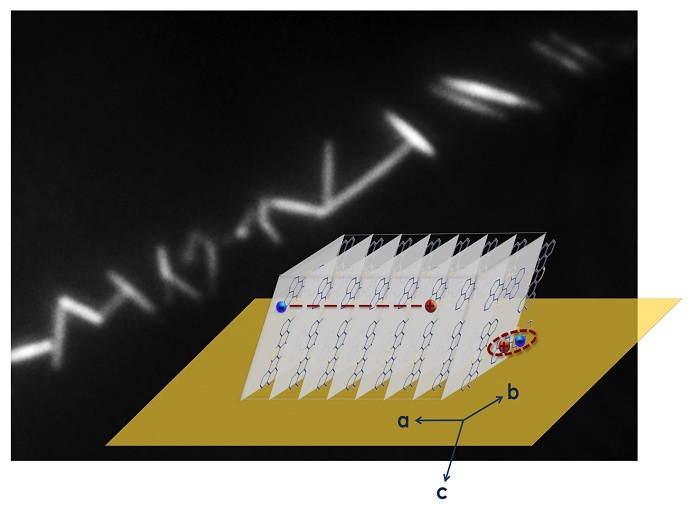
Physical chemist Michael Barnes and polymer scientist Alejandro Briseño, with doctoral students Sarah Marques, Hilary Thompson, Nicholas Colella and postdoctoral researcher Joelle Labastide, discovered the property, directional intrinsic charge separation, in crystalline nanowires of an organic semiconductor known as 7,8,15,16-tetraazaterrylene (TAT).
The researchers saw not only efficient separation of charges in TAT, but a very specific directionality that Barnes says “is quite useful. It adds control, so we’re not at the mercy of random movement, which is inefficient. Our paper describes an aspect of the nanoscopic physics within individual crystals, a structure that will make it easier to use this molecule for new applications such as in devices that use polarized light input for optical switching. We and others will immediately exploit this directionality.”
He adds, “Observing the intrinsic charge separation doesn’t happen in polymers, so far as we know it only happens in this family of small organic molecule crystalline assemblies or nanowires. In terms of application we are now exploring ways to arrange the crystals in a uniform pattern and from there we can turn things on or off depending on optical polarization, for example.”
However, the UMass Amherst team believes the property is not an oddity unique to this material, but that several materials potentially share it, making the discoveries in TAT interesting to a wide variety of researchers, Barnes says. Similar kinds of observations have been noted in pentacene crystals, he notes, which show something similar but without directionality. In this work supported by the U.S. Department of Energy and UMass Amherst’s Center for Hierarchical Manufacturing, they propose that the effect comes from a charge-transfer interaction in the molecule’s charge-conducing nanowires that can be programmed.
In the conventional view of harvesting solar energy with organic or carbon-based organic materials, the chemist explains, scientists understood that the organic active layers at work in devices absorb light, which leads to an excited state known as an exciton. In this mechanism, the exciton migrates to an interface boundary where it separates into a positive and negative charge, freeing the voltage to be used as power. “In this view, you hope that the light is well absorbed so the transfer is efficient,” he says.
In earlier work, Barnes, Briseño and others at UMass Amherst worked to control the domain size of materials to match what was believed to be the distance an exciton can travel in the time it takes to radiate, he adds. “All of this premised on idea that the mechanism for charge separation is extrinsic, that an external driving force separates the charges,” he notes. The goal had been to remove the need for that interface.”
Most recently, Briseño and colleagues reached a point in synthesizing crystals where their polymer-based devices were not performing the way they wanted, he relates. Briseño asked Barnes and colleagues to use their special measurement instrumentation to investigate. Barnes and colleagues found a structural defect that Briseño could fix. “We provided some diagnostics to him to improve their crystal growth,” Barnes says.
“From this, we noticed clues that there were some very interesting things going on, which led us to the discovery,” Barnes adds. “It’s fun when science works that way. It was a very nice mutually beneficial relationship.”
“What Nature brought us was something really much richer and more interesting than anything we could have anticipated. We thought it was going to be qualitatively similar to previous observations, perhaps different in quantitative particulars, but the real story is far more interesting. In this material, they found the way it packs crystals gives rise to its own separation, an intrinsic property of the crystalline material.”
Link