September 3, 2015
Prof. Juan de Pablo’s 20-year exploration of the unusual properties of glass began, oddly enough, with the microscopic animals known as water bears.
The creatures, which go by the more formal name of tardigrades, have a remarkable ability to withstand extreme environments of hot and cold, and even the vacuum of space. When de Pablo read about what happens when scientists dry out tardigrades, then revive them with water years later, his interest was piqued.
“When you remove the water, they very quickly coat themselves in large amounts of glassy molecules,” says de Pablo, the Liew Family Professor in Molecular Engineering at the University of Chicago. “That’s how they stay in this state of suspended animation.”
His passion to understand how glass forms in such exotic settings helped lead de Pablo and his fellow researchers to the unexpected discovery of a new type of glass.
This spring de Pablo and his collaborators at UChicago and the University of Wisconsin-Madison published their findings in the Proceedings of the National Academy of Sciences. News of the breakthrough recently went viral online. A new paper bolsters the earlier glass research, which found indications of molecular order in a material thought to be entirely amorphous and random.
“These are intriguing materials. They have the structure of a liquid, and yet they’re solids. They’re found everywhere, and we still do not understand how this process of turning from a liquid into a solid occurs,” says de Pablo.
Their results potentially offer a simple way to improve the efficiency of electronic devices such as light-emitting diodes, optical fibers, and solar cells. They also could have important theoretical implications for understanding the still surprisingly mysterious materials called glasses.
Surprisingly ordered molecules
The molecular order that the researchers found came as a big surprise. “Randomness is almost the defining feature of glasses,” de Pablo says. “At least we used to think so. What we have done is to demonstrate that one can create glasses where there is some well-defined organization. And now that we understand the origin of such effects, we can try to control that organization by manipulating the way we prepare these glasses.”
In the new paper in the Journal of Chemical Physics de Pablo and five co-authors from UChicago, Wisconsin, and France show how the vapor-deposition process can create new glassy materials by manipulating their molecular orientation.
Using vapor deposition, Wisconsin’s Mark Ediger and his team create glasses in a vacuum chamber by heating a sample material, which vaporizes, condenses, and grows atop an experimental surface.
In their latest work, the researchers compared three data sets with each other: the simplified computer model of their earlier paper; a new, much more sophisticated computer model; and the experimental results.
The similarities between the data sets are striking, notesIvan Lyubimov, lead author of the Journal of Chemical Physics study and a postdoctoral research associate in molecular engineering at UChicago. The experimental results require some interpretation of the molecular configuration because of inherent limitations of optical measurement techniques.
But in the atomic-scale simulations rendered by UChicago’s Midway Computing Cluster, “we can exactly specify the molecular configuration,” Lyubimov says. “The area of uncertainty now is whether the model is accurate or not. Running these two models allows us to improve the certainty that this mechanism which we found is probably real.”
Materials Genome Initiative
The researchers’ latest results confirmed their earlier findings. Making this all possible was funding from theMaterials Genome Initiative, which President Obama launched in 2011. The multi-agency initiative seeks to help researchers develop new materials twice as fast and at a fraction of the cost as was previously possible.
“The result is here,” de Pablo says. “We have been able to generate new glasses with new and unknown properties through this combination of experiment, theory and computation.” Pursuing development of new materials through laboratory experiments alone would be more time consuming and costly, de Pablo says.
“By adding this element of theory, we can actually answer some questions a lot sooner, understand why things happened, and now start designing and engineering materials from first principles because we have a better understanding of how the process works.”
In 2012 de Pablo became one of the first faculty members to join the Institute for Molecular Engineering.
While still at Wisconsin, he and his colleagues conducted experiments to fully document the properties of some of the molecules that tardigrades and other organisms, including some plants, use to develop their protective, glassy cocoons. This work led to a patented method—with applications in the pharmaceutical and food industries— for stabilizing proteins in bacteria or cells for long periods of time without refrigeration.
“One of the companies that has licensed the patent makes cell cultures for yogurt and makes a lot of it,” de Pablo says.

Microscopic animals inspire innovative glass research
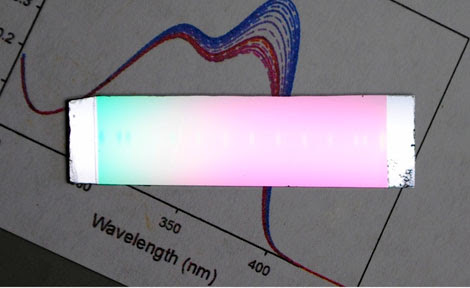
The new type of glass developed by Juan de Pablo and his associates resembles this sample, which was produced at the University of Wisconsin-Madison, in connection with a related project. (Photo courtesy of Prof. Mark Ediger/University of Wisconsin)