August 6, 2013
There may be more kinds of stuff than we thought. A team of researchers has reported possible evidence for a new category of solids, things that are neither pure glasses, crystals, nor even exotic quasicrystals. Something else.*
"Very weird. Strangest material I ever saw," says materials physicist Lyle Levine of the National Institute of Standards and Technology (NIST).
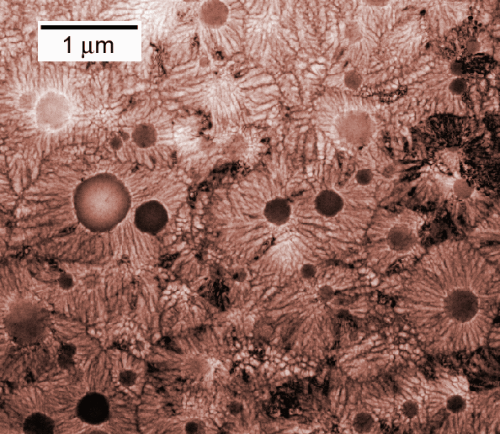
The research team from NIST and Argonne National Laboratory has analyzed a solid alloy that they discovered in small discrete patches of a rapidly cooled mixture of aluminum, iron and silicon. The material appears to have none of the extended ordering of atoms found in crystals, which would make it a glass, except that it has a very defined composition and grows outward from "seeds"—things that glasses most assuredly do not do.
The solids catalog used to be pretty straightforward. Solid stuff was either a crystal or a glass. Crystals fill up space with atoms or molecules in specific, fairly rigid patterns. The positions of the atoms are fixed such that if you take any section of pure crystal and slide it up, down, in, out or sideways a given distance, it will fit perfectly in the new position. That's translational symmetry. You can also spin the crystal through certain angles and the atoms also will line up; that's rotational symmetry.
Glasses have neither symmetry. They're just a random arrangement of their components, as if you'd taken a liquid and suddenly frozen everything in place without giving the atoms a chance to get in order. Which, in fact, is how metallic glasses are made.
In the 1980s, Dan Shechtman, an Israeli then working at NIST as a guest researcher, shook up this paradigm by finding evidence for quasicrystals, a new category of solids in which the atomic composition is fixed, and the material has rotational symmetry, but weirdly not translational symmetry. There is no long-range order to the pattern of the atoms.
The new material, which the research team has provisionally dubbed a "q-glass," can be shown by X-ray diffraction to have neither rotational nor translational symmetry, just like a glass, says Levine, but regardless, the atomic arrangement apparently is not random. "As the nodule grows, every atom still knows where to go," he says.
For one thing, the q-glass seems to have a strict chemical composition, according to Levine. Seen under a microscope, it's clear that, like a crystal, the spherical q-glass regions grow outward from a seed during cooling and exclude atoms that don't fit. "It's rejecting atoms that aren't fitting into the structure, and if there's no structure, it's not going to be doing that," says Levine. "It's amazing. Everything you can think about this thing behaves like a crystal, except it isn't."
The team used a variety of sophisticated techniques at Argonne's Advanced Photon Source to rule out other possibilities. The material might, for example, be a mass of randomly arrayed crystals so small they don't show up individually under the X-ray probes. But if such crystals were there, they'd grow slowly as the stuff is annealed. That doesn't happen. "We went through the laundry list of possibilities and disproved them, one by one," says Levine.
One possibility, say the researchers, is "frustration"—two or more incompatible crystal orderings may start growing from the seeds and continually interfere with each other, destroying any long-range order. But, "one exciting possibility is that the q-glass is the first example of a 3-dimensionally ordered configuration of atoms that possesses neither translational nor rotational symmetry," says Levine. "Such structures have been theorized by mathematicians, but never before observed in nature."
*G.G. Long, K.W. Chapman, P.J. Chupas, L.A. Bendersky, L.E. Levine, F. Mompiou, J.K. Stalick and J.W. Cahn. A highly ordered non-crystalline metallic phase. Phys. Rev. Letters 111, 015502 (2013). DOI: 10.1103/PhysRevLett.111.015502.