December 19, 2017
AMHERST, Mass. – Materials chemists have been trying for years to make a new type of battery that can store solar or other light-sourced energy in chemical bonds rather than electrons, one that will release the energy on demand as heat instead of electricity–addressing the need for long-term, stable, efficient storage of solar power.
Now a group of materials chemists at the University of Massachusetts Amherst led by Dhandapani Venkataraman, with Ph.D. student and first author Seung Pyo Jeong, Ph.D. students Larry Renna, Connor Boyle andothers, report that they have solved one of the major hurdles in the field by developing a polymer-based system. It can yield energy storage density – the amount of energy stored – more than two times higher than previous polymer systems. Details appear in the current issueof Scientific Reports.
Venkataraman and Boyle say that previous high energy storage density achieved in a polymeric system was in the range of 200 Joules per gram, while their new system is able to reach an average of 510 Joules per gram, with a maximum of 690. Venkataraman says, “Theory says that we should be able to achieve 800 Joules per gram, but nobody could do it. This paper reports that we’ve reached one of the highest energy densities stored per gram in a polymeric system, and how we did it.”
The authors say that as energy storage density improves – and with their work it is now approaching the capacity of lithium batteries – applications for the new technology include such possibilities as solar pads that collect energy from the sun by day, then store it for heating food, living spaces, clothing or blankets at night. Boyle notes that this approach will be especially valuable in areas where there is no access to a power grid.
Venkataraman says his group’s accomplishment would probably not have been possible without earlier theoretical work by Jeffrey Grossman at MIT: “Without his paper and his thoughts on the theory, I don’t think we would have gotten where we are today.” Grossman had suggested that higher energy density might be achieved if the commonly used compound, azobenzene molecules, were arranged along a rigid carbon nanotube. This frame would allow scientists to manipulate the molecular interactions, which determines how much energy is taken up and released.
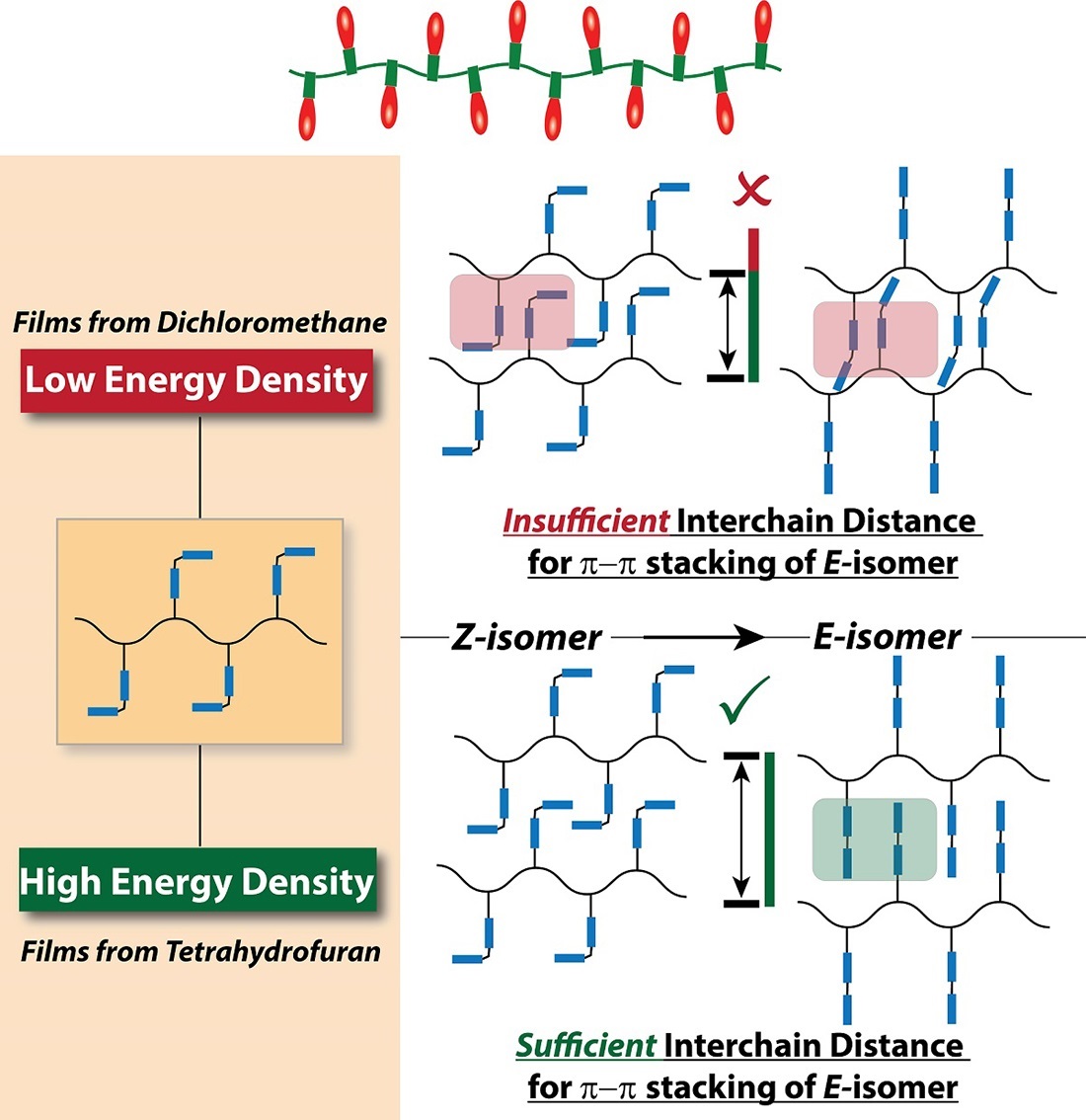
Venkataraman explains, “We understood the idea of controlling the arrangement, but we thought, What if we use a flexible polymer, not a rigid tube? Something like a string of Christmas lights, where the lights are the azobenzene molecules. Because what you cannot do with a carbon nanotube is reduce the distance between the molecules. We thought that the structure of a polymer chain would let the azobenzene groups get closer to each other and interact, which is when they gain energy and become more stable.”
Their idea worked, he adds, “but we didn’t understand why. The finding was unexpected, so we couldn’t stop there. Every time my students came to me with unexplained high numbers, I sent them back to do more control experiments to understand and validate the findings. We had to be skeptical, because we had an unusual result.”
Venkataram says, “The twist in the story is that we thought that the distance between the lights in the string was the most important. It is important, but what is more important is the way that multiple strings and their lights are carefully arranged. It turns out that the processing solvent we used acts to arrange and regulate the architecture, so the azobenzene molecules attached to the polymer are arranged very neatly and compactly. It basically acts to ensure that there can be maximum packing density.”
They used the solvent tetrahydrofuran (THF) for this processing “simply because it’s good solvent for this polymer system,” Boyle says, not suspecting that it would influence how much energy is stored and later released when we first started.
Venkataraman says, “This paper talks about how, on the molecular level, the THF affects the energy we see on the macro scale. It starts out with how the solvent molecule interacts with the polymer and it turns out that that is related to the molecular packing, how they are arranged in space. When the molecules are packed properly they can gain more energy. It took two years of work, but we finally were able to show that it’s true.”
He adds that a collaboration with scientists at Schrödinger, Inc., a scientific software and solutions company based in New York, also played a key role in helping the UMass Amherst scientists to understand the origins of the observed high energy storage densities. Led by Shaun Kwak, a lead applications scientist at Schrödinger, with experts in force-field technology Ed Harder and Wolfgang Damm, the project got the necessary company support.
Kwak says, “Working directly with scientists with experimental background at the highest level gets always marked very high in value at Schrödinger.” He emphasizes the synergetic effect he observed first-hand throughout the collaboration. “It provides a great opportunity for us to showcase the power of computational chemistry on the verge of most innovative ideas, such as shown in this work.”
The materials chemists plan to follow up this discovery with work to solve some practical problems related to charging the system, so they have not made a battery yet, but that is coming. This work was supported by UMass Amherst.
Link