September 16, 2014
The basic function of solar cells is to harvest sunlight and turn it into electricity. Thus, it is critically important that the film that collects the light on the surface of the cell is designed for the best energy absorption. The quest to develop more efficient solar cells has resulted in a fierce competition among scientists to find the lowest cost and highest energy materials.
Toward that goal, a diverse team of UCLA scientists from the California NanoSystems Institute is improving the efficiency of new film materials that are revolutionizing solar cell technology. Researchers led by Professor Yang Yang, the Carol and Lawrence E. Tannas Jr. Professor of Engineering at the UCLA Henry Samueli School of Engineering and Applied Science, recently published two studies in which they increased the power conversion efficiency of the materials kesterite and perovskite for making highly efficient and low-cost solar cells.
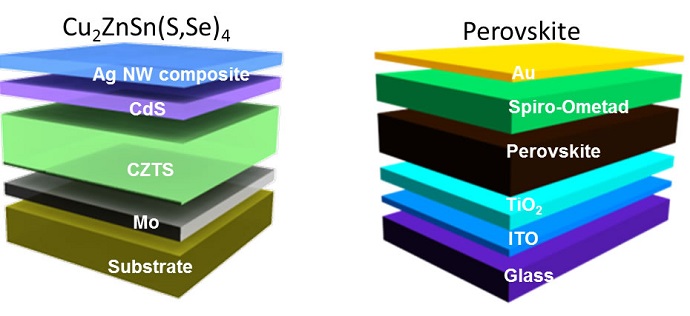
Kesterite
Kesterite is an inorganic substance (not derived from plants or animals) that is made from abundant materials, such as copper, zinc, tin and sulfur. The UCLA team has developed a way to increase the conversion of sunlight to electricity by controlling the composition and dispersion of kesterite nanocrystals in an ink that’s used to create the film used in solar cells.
In a paper published online Aug. 8 in the journal ACS Nano, the Yang group showed that their ability to control and improve the spatial composition and distribution of nanocrystals in the kesterite ink improved its power conversion efficiency to 8.6 percent with a consistent and repeatable technique.
“The device uses copper, zinc and tin, and we were able to control the ratio of the elements to make the nanocrystals better,” said Huanping Zhou, a postdoctoral scholar and first author of the study. “One problem in the past was too many defects in the film due to the element distribution problem. We are now synthesizing the nanocrystals in a way to precisely control the spatial elements and distribution in the film. This allows us to maximize solar cell efficiency.”
Yang said that the team was able to do a full solution process with the material. “That means that all the solar cell element layers needed — the adsorbent, the electrode, etc., are liquid that can be sprayed or painted on a surface to make that surface a solar cell,” he said. “That could be the roof of an electric car, or a building’s outer walls, windows or roof.”
Yang also pointed out that kesterite is very stable, and that copper, zinc and tin are inexpensive and widely available.
Perovskite
Perovskite is an organic and inorganic hybrid material that combines carbon and lead. Since it was first used as solar cell material five years ago, improvements have advanced its power conversion efficiency to nearly 20 percent, as shown in a study published in the journal Science on Aug. 1.
“We have developed a technique for controlling the formation of the perovskite to make a solar cell with just under 20 percent efficiency,” said Qi Chen, post-doctoral scholar and first author on the study with Zhou, “Perovskite is a very low cost material to produce and is very thin, one one-thousandth of the thickness of a normal silicon solar cell. It can be made flexible, hung on the wall, or could be used to build a solar farm.”
Perovskite also begins as a liquid ink, and the UCLA researchers delicately controlled the dynamics of the material during its growth, which is done in air at low temperatures. This makes manufacture of large-area perovskite devices with high performance levels inexpensive. The improved technique can be used in perovskite-based devices of such differing applications as light-emitting diodes, field-effect transistors, and sensors.
Chen said that because currently perovskite is unstable in air and deteriorates over time, the researchers are working on long-term stability to make it more stable. And because lead is a toxic element, environmentally friendly lead-free perovskite materials would be an attractive topic in the future.
Yang said that with the competition in low-cost, high-efficiency solar cells being so hot, his team pursues as many avenues as they can toward the goal of having the most efficient, lowest-cost solar cells.