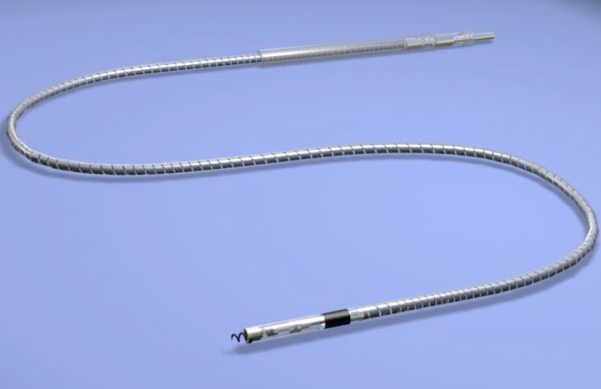
TOLOCHENAZ - May 21, 2013 -- Medtronic today announced CE Mark (Conformite Europeenne) and launch of its CapSureFix Novus(TM)5076 Lead, which is now approved in the EU for use in an MRI environment when paired with a Medtronic MR-Conditional pacemaker. The lead has not been approved for that use in the U.S.
The CapSureFix Novus 5076 Lead, previously approved for use with Medtronic's non MR-Conditional pacemakers, has long been preferred by physicians because of its small size, easy handling and proven reliability (98 percent reliability over 12 years). It is one of Medtronic's most frequently implanted pacing leads with more than 2.8 million implants worldwide.
With CE Mark, this lead now can also be paired with Medtronic's SureScan® pacing systems which are approved for use in an MRI environment. In addition, patients who previously had the leads implanted (with non-MRI pacemakers) will have the option to receive MRIs when their devices are changed at the time of battery depletion, if their replacement devices are Medtronic MR-Conditional.
"With the lead's 98 percent reliability over the past 12 years, patients and physicians can benefit from a lead that is proven to be dependable and now has the added benefit of MR compatibility," said Brian Urke, vice president and general manager of the bradycardia business at Medtronic.
The CapSureFix Novus 5076 Lead underwent extensive MRI testing - more than 1.5 million computer-modeled scenarios evaluating different patient body types, scanning locations, MRI scanner types and lead lengths - to ensure its ability to function properly in the MRI environment.
The first SureScan pacemaker system was introduced in Europe in 2008 and its use in the MRI environment is supported by clinical studies and extensive computer modeling, as well as real-world data. To date, more than 100,000 Medtronic SureScan devices have been implanted worldwide[i]. It is estimated that up to 75 percent of patients worldwide with implanted cardiac devices are expected to need an MRI scan during the lifetime of their devices.[ii]
MRI is the standard of care in soft tissue imaging, providing information not seen with X-ray, ultrasound or CT scan. MRI is therefore critical for the early detection, diagnosis and treatment of many diseases. And until recently, patients with implanted pacemakers were denied access to MRI procedures because the interaction can be harmful.[iii],[iv],[v],[vi]
This is the latest addition to a growing number of Medtronic devices which are designed for MRI access including the SynchroMed® II programmable drug infusion system available worldwide, and the SureScan® neurostimulation systems for the management of chronic pain which are available in Europe.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world.
ABOUT MEDTRONIC
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology -- alleviating pain, restoring health and extending life for millions of people around the world.
[i] Medtronic data on file. November 27, 2012.
[ii] Kalin R and Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. PACE 2005;28:326-328.
[iii] Faris OP, Shein M. Food and Drug Administration perspective: Magnetic resonance imaging of pacemaker and implantable cardioverter-defibrillator patients. Circulation 2006;114:1232-1233.
[iv] Roguin A, Schwitter J, Vahlhaus C, et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace 2008;10:336-346.
[v] Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation 2007;116:2878-2891.
[vi] Kalin R and Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. PACE 2005;28:326-328.