September 10, 2014
After the hydrogen atom, which consists of one proton and one electron, the helium atom is nature´s simplest atom. The helium atom consists of a doubly-charged nucleus surrounded by two electrons. The presence of two electrons in the atom introduces a novel aspect with profound consequences, namely the concept of electron correlation. In a paper published in Physical Review Letters on September 4, 2014 [1], experiments are reported that were performed at AMOLF where the onset of electron correlation in the helium atom is observed. Photoionization of helium is studied under conditions where the electron correlation can be turned on or off at will. When turned off, the helium atom behaves just like a hydrogen atom; when turned on, the helium dynamics is strongly affected by the interaction between the two electrons. In the experiment, helium atoms were ionized by the absorption of a single ultra-violet photon. This was possible because, prior to the experiment, the helium atoms were excited into a long-living excited state, by means of a collision with an energetic electron in a discharge source. The energy of the ultra-violet light was tuned in such a manner that it was only just sufficient for ionization of the atom, with 99.9% of the photon energy being used to overcome the ionization potential of the atom, and just 0.1% of the photon energy being converted into photoelectron kinetic energy. The very slow photoelectrons were accelerated towards a two-dimensional detector, where their position was measured. This position is then a measure of the velocity of the electron in the plane of the detector.
As clearly shown in the famous double-slit experiment on interference of single electrons (voted "the most beautiful physics experiment ",
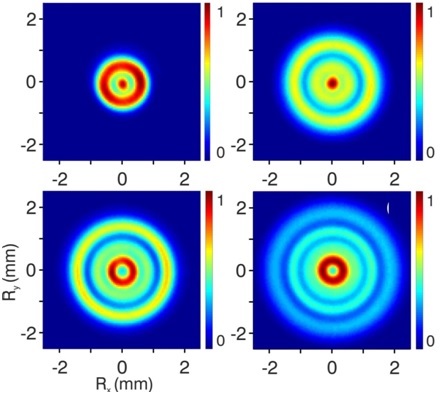
in a poll conducted by Physicsworld about a decade ago) electrons exhibit both particle- and wave-like behavior. The wave-like behavior derives from the de Broglie wavelength that quantum mechanics associates with any moving particle. The lower the kinetic energy of the electron, the larger the de Broglie wavelength is. Correspondingly, for low enough kinetic energies, the de Broglie wavelength becomes observable on macroscopic length scales. In the helium photoionization experiment the wave-like nature of the slow electrons leads to the observation of a series of interference rings, with constructive and destructive interferences alternating on the detector (see Figure 1).
All this was already known from experiments performed by the team of former AMOLF groupleader Marc Vrakking in the last decade. In fact, these experiments had revealed the existence of two distinct origins for the observed interferences. In experiments on hydrogen atoms published last year [2], it was shown that the interferences could be connected to the nodal patterns of the wavefunctions excited in the atom upon absorption of a photon. In other atoms, such as the extensively studied xenon atom, it was shown that the interferences could arise as a result of differences in the lengths of possible paths of the electron on the way to the detector. Crudely speaking, two paths differing by an integral number of de Broglie wavelengths will interfere constructively, whereas two paths differing by a half-integer number of de Broglie wavelengths interfere destructively.
In helium, both situations have now been observed to coexist. Moreover, it was observed that the helium dynamics can be controlled by tiny changes (<<1%) in the strength of the external electric field. This suffices to convert an atom that reveals the nodal pattern of its wavefunction in a hydrogen-like manner, into an atom where electron correlation removes the observability of this nodal pattern, and where the observed interference patterns are completely determined by pathlength differences between the atom and the detector. In this manner, the helium atom constitutes a wonderful nano-scale laboratory for the onset of electron correlation.
Reference
1. Stodolna, A.S., et al., Visualizing the coupling between red and blue Stark states using photoionization microscopy. Phys. Rev. Lett.113, (2014)
2. Stodolna, A.S., et al., Hydrogen Atoms under Magnification: Direct Observation of the Nodal Structure of Stark States. Physical Review Letters 110, 213001 (2013)